“It’s like time stopped,” says Emilia Bagiella, a clinical trial statistician at the Icahn School of Medicine at Mount Sinai in New York City.
When Bagiella left work one day in early March 2020, she hadn’t fully grasped how long it would be until she came back. “See you in a couple of weeks, when this is over,” she told her co-workers. But even though the reality of the pandemic had not fully hit her, a thought crossed her mind: “What’s going to happen to all of these clinical trials?”
For the next year, says Bagiella, it felt as if the clock had stopped ticking for some clinical trials, even as researchers, patients and funders fought to keep studies on track. Now that vaccines have sent the crisis phase of the pandemic into remission in some countries, researchers such as Bagiella are returning to their workplaces, finding out what impact the delays of the past year have had, and wondering what lasting lessons will be drawn from the pandemic.
“COVID-19 taught us that there’s a lot more flexibility in the clinical trials system than we realized,” says Meg Mooney, associate director of the Cancer Therapy Evaluation Program at the US National Cancer Institute (NCI) in Bethesda, Maryland. “But when you lose time, you lose time. It’s going to delay results.”
Enrolment paused
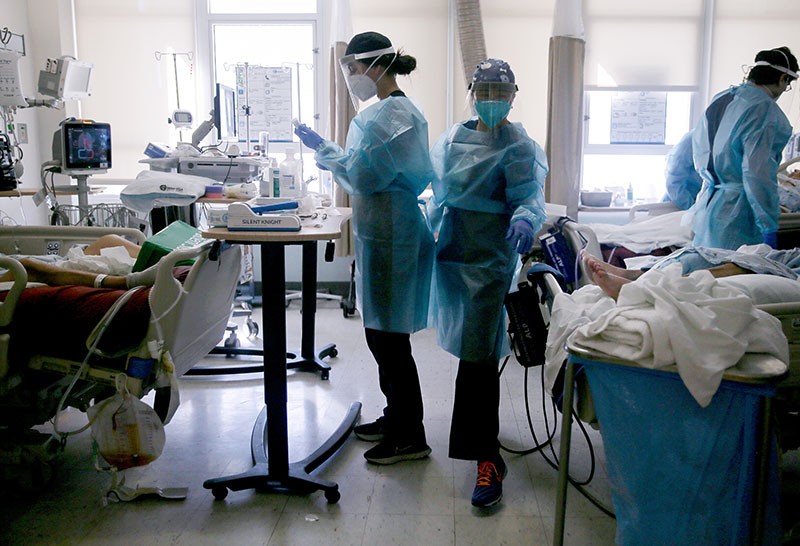
For many regions in the United States, the biggest blows to clinical research came between March and May 2020. Trial enrolments plummeted at many medical centres as prospective participants shied away from risky trips to hospital, and research staff were either furloughed or co-opted to aid hospitals’ COVID-19 treatment efforts. Some trials were deemed too dangerous to continue. Bagiella recalls the decision to pause a heart-transplant trial: transplants, and the treatments for suppressing the immune system that often come with them, were especially risky during a pandemic. She adds that “operating rooms around the country became intensive care units”, so trials involving elective heart surgery were disrupted.
Researchers worked hard to keep trials that were deemed as “life-saving” open, Bagiella says, particularly cancer-treatment studies. Even so, enrolment in clinical trials run by the SWOG Cancer Research Network, a large clinical-trials programme funded by the NCI, dropped by about half between March and May 2020. Small, early clinical trials — which often focus on establishing the safety of a new medicine — were more likely to pause enrolment than larger trials designed to demonstrate how well a therapy works against disease, Mooney found when analyzing data from two large NCI study networks.
The pandemic also took a bite out of new study launches: one study, which examined more than 62,000 trials that started before and during the pandemic, found that the number of studies initiated in the United States from February to May last year was only 57% of what would have been expected had the pandemic not occurred.1. The impact outside the United States was smaller, with 77% of the expected number of new studies launching.
Flexible arrangements
Funders and the US Food and Drug Administration responded with a series of guidelines on how clinical trials could be altered to allow them to continue during the public-health emergency. Investigators were allowed to deliver some experimental medicines to participants’ homes, rather than asking them to come to a medical centre to pick them up. Participants could use online platforms to consent to taking part in a clinical trial, rather than doing so in person. Investigators lengthened the time between doctors’ visits for some study participants and performed more of those visits remotely, using phone or video calls or online questionnaires. And participants were sometimes allowed to visit their local doctor for basic procedures and assessments, rather than travelling to a central study site.
Charles Blanke, an oncologist at Oregon Health and Science University in Portland, credits those policies with getting SWOG’s enrolment back up to near-normal levels, and keeping it there even during the country’s biggest COVID-19 surge in early 2021. “We truly believe this made a gigantic difference,” says Blanke. “And we and our patients are desperate to keep it in place.”
But that added flexibility could have come at a cost. Longer intervals between assessments might mean less data for each participant. And some studies will have to make do without medical images that they were unable to collect during coronavirus surges.
If regulators and funders are to consider making this flexibility permanent, researchers will probably need to show that the quality of clinical studies has not fallen as a result. “It’s going to be something that’s quite difficult to quantify,” says Daniel Tan, an oncologist at the National Cancer Centre Singapore, who notes that the waves of lockdowns and re-openings at varying times in different countries could complicate the analysis of international trials.
Studies to assess the impact of the pandemic on the quality of trial data are now underway. Joseph Unger, a health-services researcher and biostatistician at the Fred Hutchinson Cancer Research Center in Seattle, Washington, has been collecting questionnaires from clinical trial participants for years, to assess whether patient-reported outcomes vary if they are reported over the phone rather than in person. He hopes to use that information to gauge the effect of the remote assessments that have frequently been used during the pandemic.
Unger says that SWOG is also looking at metrics for treatment trials, and at whether the data quality has dropped off during the pandemic. “If not, then I do think that you’re going to see a lot of these adaptations become permanent,” he says.
Lasting changes
Some research centres say that the urgency of the pandemic forced them to accelerate their procedures in ways that will carry over to future trials, regardless of whether changes to official guidelines stay in place. Katherine Tuttle, a nephrologist and executive director for research at Providence Health Care in Spokane, Washington, points out that her centre can now get clinical trials running in a matter of days, rather than having to wait six weeks or more, as was typical before the pandemic. “A lot of things that we knew we were doing before, that should change, finally changed because we were in a state of emergency,” she says. “We’re not going back to doing it the old way.”
But some negative impacts could also linger. Blanke points to a survey showing that about 20% of cancer survivors are less likely to enrol in a clinical trial than they were before the pandemic2. “I do worry that there’s a core of patients who will not go on a clinical trial for the next five years,” he says.
The cutbacks in elective surgeries and other hospital services have also had a lasting effect on the tumour banks that store cancer samples for use in additional research, says Bruce Johnson, an oncologist at the Dana-Farber Cancer Institute in Boston, Massachusetts. Johnson specializes in treating lung cancer, and many clinical trials for the disease attempt to match treatments with the DNA mutations present in participants’ tumours. “They cancelled elective biopsies,” says Johnson. “And so many of our trials are based on them.”
Although elective biopsies have restarted, cancer researchers have complained that depleted supplies of patient samples have curtailed basic research. Johnson, who is an investigator on a project to characterize the individual cells in tumours, says that the project is struggling to get the samples it needs. “It’s a challenge to meet our goals,” he says.
Over the past few months, Bagiella’s team has been coming back to the office, and trials that had been pushed aside to free up resources for COVID-19 are blossoming again. The heart transplant trial that had been put on hold is enrolling participants, and research staff that were dedicated to COVID-19 studies are turning back to non-COVID conditions.
The pandemic crisis has eased so much that Mount Sinai is struggling to find enough people infected with SARS-CoV-2 to enrol in — and complete — its ongoing COVID-19 trials. “And thank God for it,” says Bagiella. She is eager to see the COVID-19 trials close, freeing up more resources for studies of other conditions. “It’s been a long, long time,” she says. “It’s good to see some of these trials coming back to life.”
"impact" - Google News
June 28, 2021 at 09:38PM
https://ift.tt/3A3DdJU
The COVID pandemic's lingering impact on clinical trials - Nature.com
"impact" - Google News
https://ift.tt/2RIFll8
Shoes Man Tutorial
Pos News Update
Meme Update
Korean Entertainment News
Japan News Update
Bagikan Berita Ini
















0 Response to "The COVID pandemic's lingering impact on clinical trials - Nature.com"
Post a Comment